
Easing epilepsy with battery power
Help is on the way thanks to long-awaited device
3/31/2014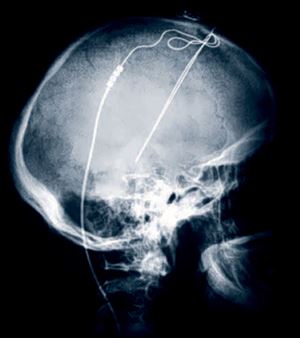
This image shows the X-Ray image of a patient with Deep Brain Stimulation leads implanted. Deep brain stimulation is routinely done for Parkinson's disease and some other illnesses, such as epilepsy.
ALEXANDRIA, N.H. — For most of his life, Kevin Ramsey has lived with epileptic seizures that drugs cannot control.
At least once a month, he would collapse, unconscious and shaking violently, sometimes injuring himself. Nighttime seizures left him exhausted at dawn, his tongue a bloody mess. After episodes at work, he struggled to stay employed. Driving became too risky. At 28, he sold his truck and moved into his mother’s spare bedroom.
Cases of intractable epilepsy rarely have happy endings, but today Ramsey is seizure-free. A novel battery-powered device implanted in his skull, its wires threaded into his brain, tracks its electrical activity and quells impending seizures. At night, he holds a sort of wand to his head and downloads brain data from the device to a laptop for his doctors to review.
“I’m still having seizures on the inside, but my stimulator is stopping all of them,” said Ramsey, 36, whose hands shake because of one of the three anti-seizure drugs he still must take. “I can do things on my own I couldn’t do before. I can go to the store on my own, and get my groceries. Before, I wouldn’t have been able to drive.”
Just approved by the Food and Drug Administration, the long-awaited device, called the RNS System, aims to reduce seizures and to improve the lives of an estimated 400,000 Americans whose epilepsy cannot be treated with drugs or brain surgery. “This is the first in what I believe is a new generation of therapy for epilepsy,” said Dr. Dileep R. Nair, head of adult epilepsy at the Cleveland Clinic and an investigator in the pivotal trial for NeuroPace’s RNS. “It’s delivering local therapy. It’s not taking tissue out; the brain is left intact. And it’s unlike a drug, which is a shotgun approach.”
Already, Nair’s center has 70 people on a waiting list for the device. Roughly 110 epilepsy centers with sophisticated diagnostic testing have filed paperwork to be able to offer it, said Dr. Martha J. Morrell, chief medical officer at NeuroPace, based in Mountain View, Calif. That represents most of the estimated 130 Level 4 centers that treat adults with epilepsy.
Unfortunately, many patients are not referred to these centers by their doctors until they have spent years, even decades, grappling with their condition.
“We want this yesterday,” said Dr. Orrin Devinsky, director of the Comprehensive Epilepsy Center at NYU Langone Medical Center. Next month, pending insurance approval, the center plans to implant the device in its first patient.
An estimated 2.3 million adults nationwide have epilepsy, and in a third of them, seizures are not controlled by drugs. Brain surgery can relieve seizures completely, but many patients aren't candidates because their seizures start in parts of the brain that can't be removed, such as those needed for language or memory.
Without treatment options, people with intractable epilepsy often find it difficult to hold jobs or to find spouses. They can suffer repeated injuries from falls and burns; their mortality rate is two to three times higher than that of the general population. "There are people out there who are just desperate for the next treatment," said Janice Buelow, vice president of research for the Epilepsy Foundation.
In a randomized clinical trial of 191 people at 32 sites, patients received stimulators but did not know whether they were activated or not. Those with stimulators activated reported a 38 percent reduction in seizures over three months, compared with a 17 percent decrease among those whose stimulators were not, according to the results published in Neurology. Over two years, 90 subjects with the devices turned on experienced a 50 percent or greater reduction in seizures.
Until he received a stimulator in 2008, Andrew Stocksdale, 32, of Mansfield, Ohio, experienced up to 20 seizures a day. By contrast, in the past month, he's had three. He is now married, holds a full-time job, and has a newborn son.
"My life fell together like a jigsaw puzzle," Stocksdale said. "I was afraid to have a son before. I couldn't do things. I was afraid of falling. I couldn't hold him."
Implantation surgery requires two days in the hospital, but extensive evaluation is necessary beforehand, including days of monitoring without anti-seizure drugs.
The device, which requires a battery change every two to three years, works only for people whose seizures start in one or two places in their brain. Electrical stimulation delivered through thin wires placed precisely at those places helps prevent an incipient seizure from spreading.
By contrast, another treatment, a vagus nerve device — which is a stimulator implanted in the chest to prevent seizures — fires "on a preprogrammed basis with no relationship to what's happening in the brain," said Devinsky of the NYU Langone epilepsy center.