
HARNESSING THE POWER OF CREATION
Stem cells breeding super-sized hope, large-scale concern
Many convinced branch of research is revolutionizing world of science
6/23/2008
A technician examines human embryonic stem cells under a microscope in the lab of University of Wisconsin-Madison researcher James Thomson.

University of Wisconsin-Madison biologist James Thomson was the fi rst to extract stem cells from human embryos.
These rats can run.
Run, rats, run.
It's not graceful movement. Not even for a rat. But it keeps them going in ways their compatriots in a nearby cage cannot match. Those rats are lame, butt-dragging, disabled -- as the running rats were not too long ago. Both groups were precisely injured. The damage to their spinal columns mimicked a kind of injury common in humans. Then, a week later, some of the rats received an injection of special cells, cells made from human embryonic stem cells. Those rats run.
Run, rats, run.
In 2006, say the researchers who made the lame rats walk, the same treatment will be tried in humans with recent spinal cord injury. The hope is to make injured men and women walk again too.
"I've had able-bodied and spinal-cord injured patients come into my lab and put the two cages in front of them -- the treated and not treated," said Hans S. Keirstead, the researcher from the University of California at Irvine doing the spinal-cord-injury work.
"Not to overdramatize it, but tears are not uncommon. It's an incredible thing."
This is the hope of stem cell technology: That packages of unique cells can cure today's incurable afflictions.
READ MORE: Harnessing the Power of Creation
While not all researchers think this or any other stem cell treatment will be tried in humans as early as 2006, or even within the next five years, it's clear these cells have captured scientific imagination. Think of a disease, and there's a scientist looking into stem cells to defeat it. But, even if wishes don't come true, even if no one is ever cured by an injection of stem cells, researchers are convinced that these cells still will revolutionize science, creating new ways to study disease, providing new clues for treatments, helping to develop new drugs, and answering basic questions about life.
What is it about these microscopic cells that engenders such super-sized hopes?
One little word: Potential.
Some five days after egg meets sperm, the human embryo forms a nearly hollow ball of about 100 cells. The cells that form the shell evolve into the placenta. But cradled inside this shell is a small bulge of cells with a different fate. These cells, called the inner cell mass, will become everything else. They are the stem cells. They will turn into muscle cells and blood cells and the 100 cell
types that make up the brain. They will become heart and thyroid and liver. In short, they can do just about anything. They've got potential.

Disabled rats regained spinal cord function after a stem-cell treatment in Hans Keirstead’s University of California at Irvine lab. Mr. Keirstead, above, also is working on treatments for MS.
Wisconsin pioneer
So did James Thomson in 1998. But he didn't have the outward trappings of it just yet.
In science, the most important researchers get the biggest laboratories. They have lots of graduate students and post-doctoral investigators working beneath them. They are the conductors of sizeable scientific orchestras.
In 1998, Dr. Thomson was more like a one-man band. The researcher at the University of Wisconsin-Madison was assisted by a single graduate student.
But he had big plans. Dr. Thomson was trying to mine the human embryo. He wanted to take it apart, grab the inner cell mass, and farm those cells into colonies of human possibility.
It had been done with mouse embryos 20 years earlier. But the human embryo proved trickier. The best mouse stem cell methods didn't work with human embryos.
His challenge was to do more than simply dissect an embryo and capture the stem cells. He also had to keep the captives from running away to meet their potential too soon. Turn your back on a plate of stem cells, and some start beating like heart muscle. They twist into networks of nerves. They spread into sheets of skin. It's what they were made to do. In nature, these cells are fleeting entities, teetering on the edge of metamorphosis. Fourteen days after egg meets sperm, they're transforming, taking their first step toward an ultimate fate. Once they take that step, a bit of cellular aptitude is lost.
Dr. Thomson was making a bid to stop time and preserve the potential of this small population of cells. First, he worked with monkey embryos. And in 1995, his team became the first to isolate stem cells from a primate. That work was federally funded.
Human embryos were a natural next step. But it was a step into a desert. Politicians had shut off federal funding to all human embryo research years earlier. Many said that destroying a human embryo, even for the most noble of purposes, is taking a life.
"It has never been, and it will never be, acceptable to kill one person for the benefit of another -- no matter how big or how promising the purported benefit," said U.S. Sen. Sam Brownback (R., Kan.), who wrote legislation protecting embryos.
But others, including U.S. Sen. Orrin Hatch, (R., Utah), an opponent of abortion, viewed embryos far differently.
"I believe that human life begins in the womb, not a petri dish or refrigerator," Senator Hatch said in 2001. "To me, the morality of the situation dictates that ... embryos, which are routinely discarded, be used to improve and extend life. The tragedy would be in not using these embryos to save lives."
So any research into human embryos depended on private money. When Geron Corp. of California offered Dr. Thomson financial support, he accepted.
Using his experience with monkey embryos, and taking advantage of new developments in fertility clinics where embryos were made, the stage was set. Dr. Thomson went to work.
In 1998, he succeeded. He derived five different stem cell lines from five of 14 embryos.
No one could guess the secrets they would tell.
Secrets of suicidal cells
If he didn't know better, Thomas Zwaka would swear that all the stem cells in the dish he watched were about to commit suicide.
Dr. Thomson's peer at the University of Wisconsin wanted to study a phenomena called programmed cell death. This is cellular suicide, a step cells take when they are no longer needed, or when genetic errors overwhelm gene repair efforts. In any dish of stem cells, Dr. Zwaka expected to find a few killing themselves. To identify them, he looked for an enzyme that suicidal cells use to chew themselves apart. But what he saw was just wrong: Every cell on the plate churned out death enzymes, but none were dying.
August, 1995: James Thomson of the University of Wisconsin -- Madison, is the first to derive stem cells from a primate embryo. He used the embryos of a South American and African monkey.
January, 1996: The Wisconsin Alumni Research Foundation applies for a patent on James Thomson s work on monkey stem cells. The patent protection may also extend to human stem cells, although in 1996 these had not been successfully harvested.
March, 1996: Great Britain s Human Fertilization and Embryology Authority issues its first license for the creation of human embryonic stem cells.
HFEA is in charge of all embryo research in England.
July, 1996: Dolly, the world's first cloned mammal, is born.
March, 1997: President Clinton bars federal funding for research into human cloning.
November, 1998: James Thomson announces that he has derived stem cells from a human embryo. John D. Gearhart of Johns Hopkins announces he has made stem cells from sperm and egg precursor cells found in fetuses.
August, 2001: President George Bush permits federal funding of research on stem cell lines created on or before Aug. 9, 2001.
February, 2004: A team of South Korean scientists derives human embryonic stem cells from the world s first cloned human embryo.
Clearly, something was fishy here. He looked for two other signs of suicide, and found those, too. Yet still no death. These cells are living with knives in their hearts, bullets in their brains, trains running them down. Yet they were just fine. Perfect.
What would happen, Dr. Zwaka wondered, if he shut down the suicide enzyme. He added a chemical known to block the enzyme's action. In two days, every cell on the plate turned into something else. They stopped being stem cells. They differentiated. They lost their potential.
It was all so topsy-turvy. The enzyme that signals a last good-bye in mature cells behaved like the fountain of youth in stem cells. It kept the stem cells from dividing as stem cells.
This discovery may end up as nothing more than a interesting footnote in the annals of embryo development, another nugget of basic science knowledge. But it could be more.
Think about cancer. There are a growing number of researchers convinced that cancer is a disease of stem cells. Genetic errors in an adult stem cell -- cells that give rise to blood cells or lung cells, for instance -- may lead to tumor formation.
For some forms of cancer, such as leukemia, the role that stem cells play is almost undisputed. For other types, evidence is mounting.
Dr. Zwaka wonders if this suggests new implications for his discovery.
"How do we treat cancer? Right now, we try to kill all the cells," he said. Chemotherapies push cancer cells to commit suicide. Sometimes it works.
But if errant stem cells cause cancer, what would happen if, instead of encouraging suicide, you block the suicide enzyme the way Dr. Zwaka did in his dish of stem cells? Maybe those guilty cancer stem cells would no longer be stem cells. Then, they'd no longer be capable of reproduction and no longer able to make cancer.
"We can look with the knowledge we acquire from embryonic stem cells and go back into other systems, like cancer," he said.
With all the hope of stem cell cures, the biggest rewards may come from their role as scientific tools.
"I believe that there is promise for these cells in the area of transplantation biology. But I also believe that the long-lasting legacy of these cells is as a basic research tool," Dr. Thomson said.
Science has seen this before. When researchers learned to engineer genes and mess with DNA, everyone predicted miraculous cures: DNA would be injected into sick cells, faulty genes replaced. Inherited diseases would be consigned to history. We'd all go to heaven with perfect bodies and barely a reason to die in the first place.
But no one predicted what really happened. Genetic engineering has been used a little more than a dozen times to treat human disease. Yet it's changed everything in science. Anthropology, drug discovery, disease diagnosis -- it's hard to think of a biological broom closet untouched by genetics.
"We had predicted that gene therapy would be successful within 10 years and nobody would have genetic diseases," Dr. Thomson said. "[We] severely underestimated how pervasive the technology would be in basic research across all sorts of things -- like an O.J. Simpson trial or your Tide [laundry detergent]. It has genetically engineered proteins in there, to make your stuff whiter. It's just everywhere. I think the same thing will be true with human embryonic stem cells."

Seeing breakthroughs
Thomas Okarma doesn't talk about stem cells as tools. He talks about cures and breakthroughs, and just how far ahead of the pack his company, Geron Corp., is. And maybe it is.
Folks who control the human stem cell patent at the University of Wisconsin, for one, think so. If Geron has a lead, it could be because it was there at the beginning.
It paid for much of Dr. Thomson's human embryo research, and it holds exclusive license to some stem cell types. Plus, it had the field almost to itself in the first three years after Dr. Thomson's discovery.
A lack of federal funding kept most U.S. university researchers away.
"They are so far behind where we are, they don't even know it," Mr. Okarma said.
Geron is also the company behind the running-rat research.
While Mr. Okarma and Mr. Keirstead -- the researcher who helped the rats run -- are high on the rat work, there are plenty of skeptics. The research has not yet been published in a peer-reviewed scientific journal. Until that happens, it is impossible for others to evaluate the claims about the rats, or the real possibility of using the research in humans soon.
Jerry Silver, a researcher in spinal chord injury at Case Western Reserve University in Cleveland, said Mr. Keirstead and Geron have made "very strong and remarkable claims" for their stem cell work. When he first heard about the research at a scientific meeting, "I was really excited,'' but, "I haven't seen it published. And that's a problem.''
The technical barriers any stem cell therapy must scale is fertile terrain for skepticism.
Fixing spinal injuries
In most spinal cord injuries, Mr. Keirstead said, it's not just the severing of a nerve that can cause paralysis. A hard blow to the spine also damages the insulation that wraps the nerves. This insulation is called myelin. Electrical signals will not travel the length of the neuron if myelin is absent.
So Mr. Keirstead's goal was to turn stem cells into a form of cell that will manufacture fresh myelin. These cells are called oligodendrocytes.
First, he had to figure out what combination of chemicals would push stem cells into becoming oligodendrocytes. Then, he had to create pure populations of these cells for transplantation. It's hard enough to figure out nature's code to perfectly transform a stem cell into another cell type. Doing it well enough so that every cell is a myelin maker is harder yet.
"There's not a single cell type in your brain anyone's ever been able to make in high purity,'' Mr. Keirstead said. "This is the first time.''
One objective is to make sure every stem cell evolves, because undifferentiated stem cells may go on to form tumors once inside the body.
The risk of tumors is why Richard Doerflinger doesn't believe this research will move into human trials next year. Mr. Doerflinger is deputy director of pro-life activities for the U.S. Conference of Catholic Bishops.
"I'll believe it when Thomas Okarma himself is the first patient,'' he said. Mr. Doerflinger said studies from other labs show an increase in tumor formation among animals that receive stem cells, even when the stem cells have differentiated, as have the cells in the rat study. "You might need just one cell in a million that's still ready to turn into anything, and you'd still have too dangerous a tendency for tumor formation,'' Mr. Doerflinger said.
Mr. Keirstead said he's never seen a tumor in any of his rats, but he said he's always working to improve detection methods so no tumor, or cell capable of becoming one, slips by.
"Safety and toxicity studies are a very humbling game,'' Mr. Keirstead said. If he doesn't see the wrong type of cell, he asks himself, "are my detection methods good enough? You have to go back and second guess absolutely everything. We are obliged to do intricate and exhaustive safety studies.''
Even if the cells are pure, there are still challenges. Will a patient's immune system attack the newly transplanted cells? The new cells will bear the genetic signature of the embryo that made them. That could make trouble.
Mr. Okarma said such immune rejection will be prevented with mild doses of immune-suppressing drugs. He's counting on studies that suggest that stem cells don't excite the immune system the way mature cells do.
Researchers are also concerned with contamination: many stem cell lines grow on a layer of mouse cells, as were the running-rat cells. Some worry these mouse feeder layers, which provide the stem cells with essential nutrients, could contaminate human subjects with mouse pathogens.
But Mr. Okarma says this is not a significant problem. The U.S. Food & Drug Administration doesn't prohibit the use of stem cells grown on mouse cells, although it does require testing and other precautions. Plus, Geron has created additional stem cell lines that have never been exposed to mouse cells.
Mr. Keirstead said his work with myelin-making cells may eventually provide treatment in another disease characterized by myelin loss: multiple sclerosis. "It's important to note we're not there yet,'' he said. "With spinal cord injury, we are very close."
While spinal cord injury can, theoretically, be treated with an injection to the damage site, MS is a diffuse disease, occurring randomly around the nervous system. So there's a problem even getting the myelin-makers to the injury sites. Then, there's the scarring that accompanies the loss of myelin in MS. Once scars set in, whether in spinal cord injury or MS progression, stem cells won't work.
"We have to go in before they scar, or we have to develop a means to get rid of the scar,'' Mr. Keirstead said. "At least we've got a tool now.''

A technician examines human embryonic stem cells under a microscope in the lab of University of Wisconsin-Madison researcher James Thomson.
Opening door to research
In 2001, Idelle Datlof of Cincinnati said good-bye to her 30-year career as a mental health professional. She couldn't work anymore. The multiple sclerosis she was diagnosed with at 33 sent her home.
MS is known as a relapsing-remitting disease. Its victims may be graced with long periods of good health, then rocked by sudden, irreversible declines. This is the crooked highway Ms. Datlof has followed for 26 years.
"I was diagnosed in 1978 -- a long time ago. I have diligently paid attention to all the research advances. There have been very few,'' Ms. Datlof said. "And with all of them, there have never been words like repair and regeneration. Those are astonishing words for this condition."
She was stunned to hear those words in 2001 in discussions of stem cells. Finally, some hope. She formed an advocacy and educational effort called Stem Cell Action Network -- which today has about 1,000 members -- and joined what is perhaps an unprecedented effort in support of a scientific technology.
A lot of people first heard of stem cell research in 2001, when President George Bush announced a compromise agreement to allow federal funding for research on some embryonic stem cell lines. Prior to his action on Aug. 9, 2001, no federal money went to any research on human embryos, although President Clinton's administration was proceeding with plans to fund stem cell research when Mr. Bush took office.
Mr. Bush's decision was an attempt to walk a fine line between scientific progress and moral rectitude.
The president said the federal government would fund research using stem cell lines "where the life-and-death decision has already been made." The government would not support any work that required the destruction of more embryos. No research on stem cell lines created after his Aug. 9 announcement would be eligible for funding.
As with most compromises, it brought complaints from all sides. For those who believed destroying an embryo was the equivalent of taking a human life, it sanctioned immoral research.
Those who sought greater research into stem cells complained that the president's decision was too limiting. Dr. Thomson, who first derived human stem cells, takes a realpolitik view on the President's decision.
"It's bad and we need to go beyond it, but it did open the window to doing this research.''
It is especially important that the compromise was made by a Republican president, he said.
"It gave researchers the confidence that, even if the administration changed, it would keep being funded.'' If a Democrat had approved stem cell funding, "There would still be this fear in the back of people's minds that if a conservative Republican were elected, it would all be stopped.''
In a sense, Mr. Bush's decision opened the door a crack, and a battalion of stem cell advocates rushed forward to make the opening even wider -- from mothers with diabetic children, to Hollywood stars such as Michael J. Fox and the late Christopher Reeve.
"I don't know that there's ever been so much attention paid to an area of medical science,'' said Lawrence Soler, vice president for government relations with the Juvenile Diabetes Research Foundation, and founder of an umbrella organization for stem cell advocates, the Coalition for the Advancement of Medical Research.
The advocacy has an impact. Most polls on embryonic stem cell research show that some 70 percent of Americans favor its development, he said. There are a growing number of congressmen taking up the issue, including U.S. Sen. Arlen Specter (R., Pa.), who has been fighting on behalf of this research from the start, and U.S. Rep. Mike Castle (R., Del.), whose leading an effort for stem cell funding in the House of Representatives.
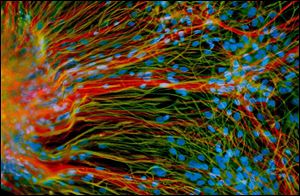
These neural cells started life as embryonic stem cells. Here they generate mature neurons (red) and glial cells (green) in a laboratory at the University of Wisconsin-Madison.
Adult vs. embryonic cells
Amit Patel wanted to help heart failure patients. So the University of Pittsburgh cardiac surgeon injected some of his bypass patients with stem cells.
These were people with incredibly inefficient hearts. To be considered healthy, the heart's main pumping chamber must pump 55 percent of the blood it holds with each beat. Dr. Patel's patients squeezed out a mere 30 percent. Six months after the surgery, all had improved. But it was the stem-cell patients who had the best results. Their pumping efficiency increased 10 to 15 percent -- twice the increase seen with bypass alone.
"It's still not normal, but for someone in heart failure, that's a dramatic improvement,'' Dr. Patel says.
But here's the twist: Dr. Patel wasn't using stem cells from the human embryo. He used stem cells from adult bone marrow. When arguments over stem-cells get going, someone inevitably asserts that there is no need for embryonic stem cells, with the ethical issues they raise, when adult stem cells are so promising.
"This is not a debate in the scientific community,'' said Dr. Thomson. "It's a stupid question,'' he said. He characterized it as the equivalent of researchers arguing whether mice, fruitflies, or rats make better subjects for scientific study. It depends on what's being researched.
The big question about adult cells is one of potential. These are cells that have already made some decisions, commitments that may be irreversible. Blood stem cells make blood. Skeletal muscle stem cells make skeletal muscle. It's not clear that these cells can switch their programming, that a blood stem cell could make bone or a bone stem cell make brain.
Mr. Okarma of Geron Corp. dismisses adult stem cell work. "My first company was an adult stem cell company. One by one the projects got canned,'' he said. Adult stem cells are stuck with their fate, he insists. Put in the body, they don't change, they simply fuse with surrounding cells.
Dr. Patel isn't arguing that. In fact, he's pretty sure his bone marrow stem cells did not turn into heart cells, but stimulate healing in other ways.
"What we believe is, up to 30 percent of these cells may be fusing with existing cells in the tissue,'' he said. The rest of the stem cells die. Those that fuse may stimulate factors that help repair the heart.
He's designed a new study that will address the issue in more detail. He'll inject stem cells into the hearts of patients waiting for transplant. When the transplant takes place, he'll be able to study the heart tissue and learn the fate of the stem cells.
Thomas Okarma aside, there are clearly a number of people who find adult stem cells -- including fetal cells, and umbilical cord blood cells -- tantalizing. This year, the National Institutes of Health spent seven times more money on these cells than embryonic cells. Adult cells received $190.7 million; embryonic cells $24.8 million.
The fact is, it's impossible to say where the next important discovery will come from, and for people who need cures, it doesn't matter. What matters, they say, is that research move full-speed ahead on all kinds of stem cells, adult and embyronic. Success with even one embryonic stem cell treatment could be all it takes to increase funding there as well.
"All you need is one nice young person getting out of his wheelchair, and I think there's going to be an absolute uproar,'' of people demanding more research, said Ms. Datlof of the Stem Cell Action Network.
"Forget about the human suffering,'' she said. "Look at what is lost to our nation, these children and their potential. We need a new mind set."