
Toledoans tell Congress of tainted-heparin grief
4/30/2008
Johanna Staples, right, is hugged by daughter C.J. Leavens.
WASHINGTON The widow of former Toledo radio personality Dennis Staples, who died after receiving contaminated heparin made with ingredients from China, urged a congressional panel yesterday to pass the laws needed to secure a safe U.S. drug supply.
Brushing away tears, Johanna Staples told the House Energy and Commerce subcommittee on oversight and investigations that Americans have a false sense of security about the reliability of drugs. Minutes later, FDA officials told the panel they are confident that the heparin supply in the United States is now safe despite the challenges of handling a global pharmaceutical supply chain.
Mrs. Staples was joined on the panel by Leroy Hubley, who lost his wife of 48 years, Bonnie, and his son, Randy, within about a month of each other. Similar to Mr. Staples, both had undergone hemodialysis at a Toledo clinic and were given heparin, a blood thinner widely used during open-heart surgery and dialysis. Baxter International Inc. later recalled the drug.
Now I am left to deal not only with the pain of losing my wife and son, but anger that an unsafe drug was permitted to be sold in this country, Mr. Hubley said in the packed Rayburn House Office Building hearing room, his voice frequently choking with sobs.
Mrs. Staples and Mr. Hubley are among several Toledoans who recently filed wrongful-death lawsuits against Baxter International Inc. over tainted heparin. A judge is expected to decide in late May whether to consolidate all similar lawsuits nationwide in Toledo.
At yesterday s hearing, subcommittee members faulted the Food and Drug Administration and the drug companies involved in the production of heparin, and said they are working on bipartisan legislation to improve FDA funding and fix the nation s broken drug-safety system. They applauded Mrs. Staples and other Toledoans testifying before the panel, saying they put a human face on what happens when safeguards fail.
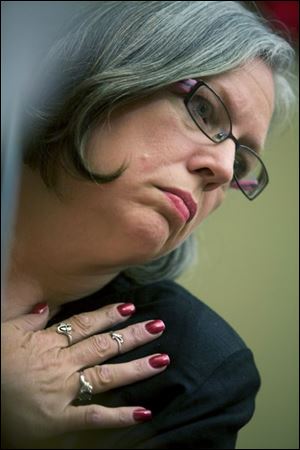
Toledoan Johanna Staples recounts the death of a loved one from tainted heparin during a hearing on Capitol Hill before a House Energy and Commerce subcommittee.
Rep. John Dingell (D., Dearborn), chairman of the House Energy and Commerce Committee, said the FDA must bolster its efforts to inspect overseas plants that make drugs for the U.S. market. He said the FDA is more trusting than a kindergarten class in assuming that the agency will otherwise be able to stop unsafe pharmaceuticals from crossing U.S. borders.
Rep. Bart Stupak (D., Mich.), the subcommittee s chairman, cited 81 deaths and at least 785 severe allergic reactions associated with heparin since January, 2007. He said 62 of the deaths occurred between November, 2007, and February, 2008.
Randy Hubley s widow, Colleen Hubley, sat beside her father-in-law and testified that her husband suffered diarrhea, abdominal pain, and difficulty in breathing following his dialysis treatment in January. Mrs. Hubley, who is a dialysis nurse, said she called 911 and performed CPR until paramedics arrived, but emergency efforts failed and she said she watched my husband and best friend slip away before my eyes. She said some of her dialysis patients suffered uncommon side effects during dialysis this winter that in retrospect might have been linked to contaminated heparin.
Baxter, which produces about 50 percent of the heparin used in the United States, initiated a nationwide recall of nine lots of heparin on Jan. 17, 2008, after an increase in adverse reactions among patients given the product.
The FDA began an investigation and found that heparin made by a Chinese manufacturing plant, Changzhou SPL, contained a contaminant called oversulfated chondroitin sulfate, which mimics real heparin at far lower cost and is difficult to find in standard tests.
At yesterday s hearing, Democratic and GOP subcommittee members said because oversulfated chondroitin sulfate is not normally found in nature and must be produced by chemical modification, evidence suggests that the contaminant was intentionally introduced into the supply chain.

Johanna Staples, right, is hugged by daughter C.J. Leavens.
This is thuggery. This is thievery, said Rep. Michael Burgess (R., Texas), a subcommittee member.
Think of the poor individual who actively administered the shot [of contaminated heparin], added Dr. Burgess, a physician. They ll carry this knowledge around forever as well.
Lawmakers pointed out the complexity of heparin production. Heparin, derived from pig intestines, has been marketed in the United States since the 1930s.
Baxter, the final manufacturer of the tainted heparin, has an international supply chain that begins with about a dozen Chinese workshops where crude heparin is produced and then sold to brokers or sold directly to a couple of companies which consolidate the product.
Consolidators sell crude heparin to U.S.-based Scientific Protein Laboratories, which has a joint venture with a plant in Changzhou, China, and also has a plant in Wisconsin that refines crude heparin into an active pharmaceutical ingredient. This in turn is sold to Baxter, which makes finished heparin products at its plant in Cherry Hill, N.J.
Robert L. Parkinson, chief executive officer of Baxter International, testified that company officials are greatly concerned that our heparin product appears to be the target of a deliberate adulteration scheme. He cited the challenges arising from the complexity of the global drug supply chain, as did David Strunce, president of Scientific Protein Laboratories.
Mr. Strunce testified that his company conducted tests showing that oversulfated chondroitin sulfate was present in certain batches of crude heparin before they reached Scientific Protein Laboratories or the Changzhou SPL factory.

Toledo radio personality Dennis Staples died in December.
Mr. Stupak said the FDA made one glaring and fatal mistake in approving Changzhou SPL to sell heparin to Baxter without first conducting a preapproval inspection of its production plant.
Lawmakers further noted that Baxter didn t conduct its first inspection of the Changzhou plant until September, 2007, a few years after starting to use heparin ingredients made there, and found only a single, minor problem. But when the FDA inspected the same plant in February, 2008, federal regulators determined that the plant was incapable of providing safe heparin to the United States and prohibited further importation of its heparin product to the U.S. pending a reinspection.
Mr. Stupak and others blasted FDA officials over the agency s decision to issue an import alert only on heparin from the Changzhou SPL plant when in fact the agency has identified a dozen Chinese sources of heparin contamination. They said it seemed unreasonable to expect 90-some FDA inspectors covering more than 300 U.S. ports to catch drug-safety problems.
The FDA s Janet Woodcock, director of the agency s Center for Drug Evaluation and Research, said the agency is putting more FDA staff in China to inspect pharmaceutical plants and to interact more with manufacturers to ensure that products shipped to the United States meet U.S. standards.
Mrs. Staples told the committee in a written statement that Baxter needs to be held accountable as well as the FDA.
This drug surely helped to increase Baxter s corporate bottom line, she said, noting that Baxter s U.S. sales totaled more than $4.8 billion last year, with global net sales of $11.3 billion.
After his testimony, Mr. Hubley, surrounded by family members, said outside the hearing room: I think they did a fine job. You kind of lose your faith in government these days, but who knows? They acted very sincere.
Contact Judy Packer-Tursman at: tursman@comcast.net.